Introduction
R-CHOP (rituximab, cyclophosphamide [CY], doxorubicin [DXR], vincristine [VCR], and prednisolone) is the standard of care for diffuse large B-cell lymphoma (DLBCL) and cures around 60% of patients. However, some DLBCL survivors suffer from long-lasting peripheral neuropathy (PN) caused by VCR, and the dose of VCR is usually decreased to prevent severe PN. Though, there is a lack of information regarding the effect of VCR dose reduction on DLBCL prognosis, especially based on data from prospective clinical trials.
Recently, VCR dose reduction was not associated with poor prognosis in patients with aggressive B-cell lymphomas, based on the results of the RICOVER-60 trial (NCT00052936) (Bewarder et al., Haematologica 2023). In this trial, R-CHOP was administered in 14-day cycles. To evaluate the clinical impact of relative dose intensity (RDI) of VCR (RDI O) in patients with DLBCL treated with tri-weekly R-CHOP (R-CHOP21), we conducted a supplemental analysis of the JCOG0601: randomized phase II/III trial of the Japan Clinical Oncology Group (jRCTs031180139). In the JCOG0601 trial, RW-CHOP21 (CHOP21 with eight doses of weekly rituximab) and R-CHOP21 were compared for progression-free survival (PFS) (Ohmachi et al., Blood Adv. 2021).
Methods
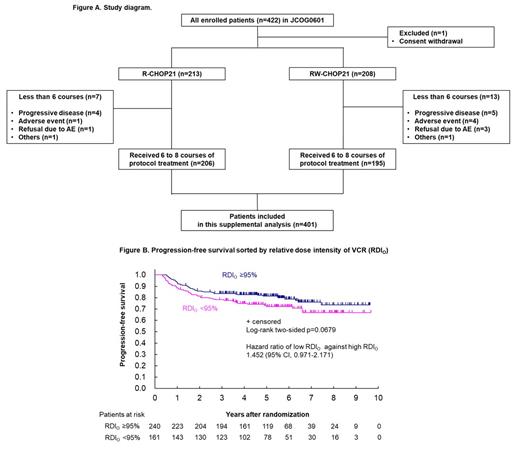
Between 2007 and 2014, 422 patients were enrolled in the JCOG0601 trial. Among them, 401 patients who received at least six courses of R-CHOP21 (n=206) or RW-CHOP21 (n=195) were eligible for this supplemental analysis. Those who could not complete six courses of chemotherapy were excluded because their treatment outcomes might be impaired mainly due to their disease aggressiveness and not the dose intensity of chemotherapy. As PFS was not significantly different between the two treatment arms, these 401 patients were analyzed together (Figure A).
The impact of decreased RDI O on PFS was assessed using the Cox proportional hazard model and two-sided log-rank p-values with several cutoff values of RDI O (95, 90, 85, 80, 70, 60, or 50%). The VCR dose was reduced according to the protocol. As a subgroup analysis, the impact of RDI O was evaluated in patients whose RDI of CY and DXR (RDI C+H) were retained, defined as RDI C+H were both ≥85% (high RDI C+H), or not retained, defined as either or both of RDI C+H were <85% (low RDI C+H). Clinical factors, including the cell of origin (COO), as defined by the Hans algorithm, were evaluated for their association with poor PFS.
Result
The baseline clinical characteristics of the 401 patients were as follows: median age, 62 years (range, 20-79), male sex (54.6%); performance status of 0 or 1 (97.5%); Ann Arbor stage Ⅰ or Ⅱ (53.4%); and International Prognostic Index (IPI) of low or low-intermediate (82.6%). The median RDI O of the 401 patients was 97% (interquartile range [IQR], 89-100). The median RDI of CY and DXR were as high (96% each).
At the data cut-off date (Dec 2017), the median follow-up time was 59.4 months, and the 3-year PFS was 81.5% (95% confidence interval [CI], 77.3-85.0). Among the cutoff values investigated, 95% of RDI O (<95% [n=161], ≥95% [n=240]) showed the smallest p value (log-rank test, p = 0.0679), and the hazard ratio (HR) of low RDI O against high RDI O was 1.452 (95% CI, 0.971-2.171) (Figure B); RDI O <95% clinically means VCR dose was reduced in one or more times. A cutoff value of 95% was used for further analyses. Patients with RDI O <95% tended to have low RDI C+H. According to RDI O of <95% vs. ≥95%, there was a similar difference in the subgroup of patients with high RDI C+H as in the whole population (n=324, HR 1.508, 95% CI 0.934-2.434), but no remarkable difference in low RDI C+H (n=77, HR 0.932, 95% CI 0.432-2.009). Multivariable analysis revealed that the presence of B symptoms (HR 1.859, 95% CI 1.078-3.207) and high-intermediate or high IPI scores (HR 1.721, 95% CI 1.031-2.873) were risk factors for PFS. However, neither RDI O <95%, low RDI C+H, nor COO subtypes showed no remarkable impact on poor PFS in this patient population.
Conclusion
Our study demonstrated that among patients with DLBCL who completed six or more courses of R-CHOP21, VCR dose reduction was not associated with poor PFS as much as the presence of B symptoms and high IPI risk. VCR dose reduction driven by toxicity might not impair its efficacy if patients can complete R-CHOP21 therapy.
Disclosures
Suzuki:SymBio Pharmaceuticals: Honoraria; BMS: Honoraria; Janssen: Honoraria; Sanofi: Honoraria; Astellas: Honoraria; Chugai Pharma: Honoraria. Shimada:Bristol-Myers Squibb, Otsuka, Kyowa Kirin: Research Funding; AstraZeneca, Eisai, Takeda, Janssen, Bristol-Myers Squibb, Chugai, Kyowa Kirin, Nippon Shinyaku, Daiichi Sankyo, Meiji Seika Pharma, Ono, AbbVie, Novartis, Gilead, CSL Behring, Genmab: Honoraria; Incite, Daiichi Sankyo, AbbVie, Chugai, Meiji Seika Pharma, Bristol-Myers Squibb, Novartis: Consultancy. Kobayashi:Bristol Myers Squibb, Chugai Pharmaceutical, Nippon Shinyaku, Ono Pharmaceutical, Sanofi, Nippon Kayaku, AstraZeneca, Abbvie, MSD, and Janssen Pharmaceutical: Honoraria. Maruyama:Eizai: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Taiho: Research Funding; Chugai Pharma: Honoraria, Research Funding; Amgen Astellas Biopharma: Research Funding; Kyowa Kirin: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Research Funding; Janssen: Honoraria, Research Funding; Otsuka: Research Funding; Astellas: Research Funding; Abbvie: Honoraria, Research Funding; MSD: Honoraria, Research Funding; Ono Pharmaceuticals: Honoraria, Research Funding; Nippon Shinyaku: Honoraria; Mundipharma: Honoraria, Research Funding; Zenyaku: Honoraria; SymBio Pharmaceuticals: Honoraria; AstraZeneca: Honoraria. Munakata:Janssen, Takeda, Celgene, ONO PHARMACEUTICAL, Eisai, CHUGAI, Novartis Pharma, Bristol-Myers Squibb, AstraZeneca, SymBio Pharmaceuticals, Genmab, NIPPON SHINYAKU, Nippon Kayaku, Gilead Sciences, Otsuka Pharmaceutical, Kyowa Kirin: Honoraria, Research Funding. Ohmachi:Novartis Pharma: Honoraria; Meiji Seika Pharma: Honoraria; Chugai pharma: Honoraria; Kyowa Kirin: Honoraria; Genmab: Honoraria; Symbio pharma: Honoraria; Yakuzemi total learning: Honoraria; Janssen Pharma: Honoraria. Kinoshita:Japanese Red Cross, Director of Aichi Blood Center: Current Employment. Ando:Nippon Shinyaku: Honoraria, Research Funding; Takeda Pharmaceutical: Research Funding; Chugai Pharmaceutical: Research Funding; Eisai: Honoraria, Research Funding; Kyowa Kirin: Research Funding; Otsuka Pharmaceutical: Research Funding; Novartis: Research Funding; BMS: Honoraria, Research Funding; Asthelas: Research Funding; Janssen: Honoraria; Meiji seika pharma: Honoraria; Nippon kayaku: Honoraria. Nagai:Takeda: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Eli Lilly: Honoraria, Research Funding; Beigene: Research Funding; Genmab: Honoraria, Research Funding; HUYA: Research Funding; Kyowa Kirin: Honoraria, Research Funding; MSD: Honoraria, Research Funding; Mitsubishi Tanabe: Research Funding; Chugai: Honoraria, Research Funding; Daiichi Sankyo: Research Funding; BMS: Honoraria; Zenyaku Kogyo: Research Funding; Solasia: Research Funding; Ono: Honoraria, Research Funding; Eisai: Honoraria; Novartis: Honoraria; Sumitomo Pharma: Honoraria; Meiji Seika Pharma: Honoraria; Mundi pharma: Honoraria; GSK: Honoraria; Celgene: Research Funding; Astra Zeneka: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal